Table of Contents
The pharmaceutical industry stands at the verge of a revolution. Traditional drug development goes up to a grueling 10-15 year journey costing upwards of $2.6 billion per approved drug and it is being fundamentally reimagined through generative artificial intelligence.
As we navigate through 2025, Gen AI healthcare is no longer a futuristic concept but a present-day reality reshaping how we discover, develop, and deliver personalized treatments.
In 2025, AI drug discovery has moved beyond experimental phases into mainstream pharmaceutical development. Companies are leveraging GenAI in drug development to design novel molecules, predict patient responses, and create treatments tailored to individual genetic profiles. This isn’t incremental progress—it’s a complete transformation of healthcare delivery.
The numbers started telling a compelling story. The global AI in drug discovery market is projected to reach $4.9 billion by 2025, with generative AI models now capable of screening billions of molecular combinations in days rather than decades.
For patients, this means faster access to breakthrough treatments. For healthcare systems, it promises more effective interventions with fewer side effects.
Let’s explore more about this.
How Generative AI Accelerates Personalized Medicine In 2025?
Generative AI has emerged as the powerhouse behind personalized medicine’s rapid evolution.
Unlike traditional AI systems that simply analyze data, generative models create entirely new possibilities—designing drug candidates, predicting protein structures, and mapping treatment pathways specific to individual patients.
1. Molecular Design At Unprecedented Speed
The most transformative application of GenAI healthcare & AI drug discovery lies in molecular generation. Platforms like AlphaFold 3 and proprietary pharmaceutical AI systems can now predict protein structures with near-atomic accuracy, enabling researchers to design drugs that bind precisely to disease targets. What previously required years of laboratory experimentation now happens in computational environments within weeks.
Generative Adversarial Networks GANs and transformer models analyze vast chemical libraries, then it starts learning the pattern that makes drugs effective.
2. Patient Specific Treatment Optimisation
The generative AI impact on personalized medicine extends far beyond the laboratory. In clinical settings, AI models analyze patient genomics, medical history, lifestyle factors, and real-time biomarkers to recommend treatments with the highest probability of success.
For cancer patients, this means treatment plans based not just on tumor type but on the specific genetic mutations driving their disease.
AI systems cross-reference millions of research papers, clinical trials, and patient outcomes to identify drug combinations that have worked for genetically similar patients—even if those combinations were never formally studied together.
3. Accelerated Clinical Trial Design
GenAI in drug development is revolutionizing clinical trials through intelligent patient stratification and adaptive trial design. AI models identify the most suitable candidates for trials by analyzing genetic markers, ensuring that studies enroll patients most likely to respond. This reduces trial sizes, cuts costs, and accelerates regulatory approval timelines.
Synthetic control arms created using AI to model how patients would respond to standard treatments are reducing the need for large placebo groups, making trials more ethical and efficient.
4. Real World Evidence Integration
By 2025, generative AI systems integrate real-world evidence from electronic health records, wearable devices, and patient-reported outcomes. This continuous learning loop allows AI models to refine treatment recommendations based on what’s actually working in diverse patient populations, not just controlled trial environments.
The result? Personalized medicine that adapts as quickly as diseases evolve, with treatment protocols that learn from every patient interaction.
Benefits Of AI Powered Genomics In Healthcare
The convergence of AI drug discovery and genomics has unlocked capabilities that seemed like science fiction a decade ago.
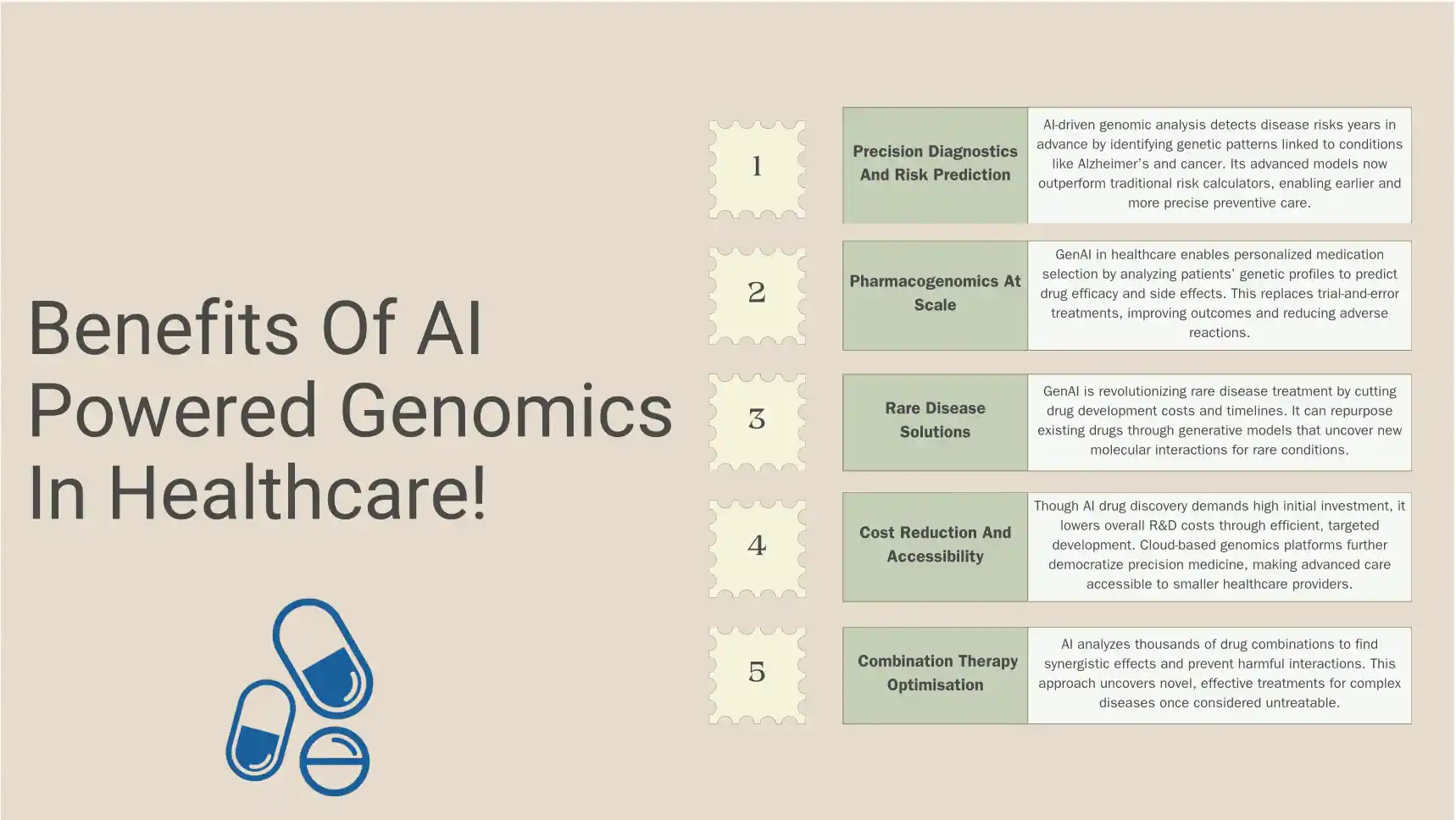
These benefits are transforming healthcare delivery across multiple dimensions.
1. Precision Diagnostics And Risk Prediction
AI-powered genomic analysis can identify disease risks years before symptoms appear. Machine learning algorithms scan genetic sequences for patterns associated with conditions like Alzheimer’s, cardiovascular disease, and rare genetic disorders. This early detection enables preventive interventions when they’re most effective.
For hereditary cancers, AI systems analyze BRCA mutations and hundreds of other genetic variants simultaneously, providing nuanced risk assessments that guide screening schedules and preventive surgeries.
The accuracy of these predictions has improved dramatically, with some AI models now outperforming traditional risk calculators by significant margins.
2. Pharmacogenomics At Scale
One of the most immediate benefits of Gen AI healthcare and AI drug discovery is optimized medication selection based on genetic profiles. Pharmacogenomic AI analyzes how individual patients metabolize drugs, predicting efficacy and adverse reactions before prescriptions are written.
This eliminates the trial-and-error approach that has historically characterized mental health treatment, pain management, and many chronic disease therapies. Patients receive medications that work with their unique biology, reducing side effects and improving adherence.
3. Rare Disease Solutions
For the 400 million people worldwide living with rare diseases, GenAI in drug development offers unprecedented hope. Traditional pharmaceutical economics made rare disease research financially unviable. AI changes this equation by dramatically reducing development costs and timelines.
Generative models can repurpose existing drugs for rare conditions by identifying unexpected molecular interactions.
They can also design targeted therapies for ultra-rare genetic mutations affecting only handfuls of patients—scenarios where traditional drug development would never proceed.
4. Cost Reduction And Accessibility
While AI drug discovery requires significant upfront investment, it substantially reduces the overall cost of bringing medications to market. Fewer failed trials, shorter development cycles, and more targeted therapies mean lower R&D expenditures—savings that can translate to more affordable treatments.
AI-powered genomics also democratizes access to precision medicine.
Cloud-based platforms allow smaller healthcare facilities to access sophisticated genomic analysis without massive infrastructure investments, expanding personalized medicine beyond elite academic medical centers.
5. Combination Therapy Optimisation
Cancer and complex chronic diseases often require multiple medications working in concert. AI systems evaluate thousands of potential drug combinations, predicting synergistic effects and avoiding dangerous interactions.
This computational approach has identified effective combinations that human researchers would never have tested, opening new treatment pathways for previously intractable conditions.
Challenges For AI In Precision Medicine Adoption
Even if AI has the potential to revolutionise the drug discovery process. But, it also does come with its own obstacles and that must be addressed so the technology can fulfil its promises.
1. Data Quality and Standardization Issues
AI models are only as good as the data they’re trained on. Healthcare data remains fragmented across incompatible systems, with inconsistent terminology, missing information, and quality issues.
Genomic data presents additional challenges—sequencing errors, population biases, and incomplete variant annotations can lead AI systems to incorrect conclusions.
The lack of standardized data formats across institutions means AI models trained on one hospital system’s data may perform poorly when applied elsewhere. Creating interoperable data infrastructure remains a massive undertaking requiring coordination across thousands of healthcare organizations.
2. Regulatory Uncertainty And Validation Requirements
Regulatory frameworks haven’t kept pace with AI innovation. Questions persist about what level of validation AI-designed drugs require, how to evaluate continuously learning algorithms, and who bears liability when AI recommendations lead to adverse outcomes.
The FDA and other regulatory bodies are developing guidelines, but the rapid evolution of generative AI creates moving targets.
Pharmaceutical companies face uncertainty about whether their AI-assisted development processes will meet regulatory standards, creating risk aversion that slows adoption.
3. Algorithmic Bias And Health Equity Concerns
Training data for AI models overrepresents certain populations primarily individuals of European descent while underrepresenting others. This creates AI systems that may recommend suboptimal treatments for underrepresented groups, potentially exacerbating existing health disparities.
Genomic databases skew heavily toward specific populations, meaning AI drug discovery efforts may inadvertently design therapies that work well for some genetic backgrounds while being less effective or even harmful for others.
4. Privacy And Security Concerns
Genomic data is uniquely sensitive, it identifies not just individuals but their blood relatives and can never be changed if compromised. Patients worry about genetic discrimination by insurers or employers, creating reluctance to participate in AI-driven personalized medicine programs.
Securing AI systems against cyberattacks poses escalating challenges as models become more complex and data repositories grow larger.
5. Cost And Infrastructure Barriers
Implementing AI drug discovery platforms requires significant computational resources, specialized talent, and ongoing maintenance. Smaller pharmaceutical companies and research institutions may lack the capital to compete, potentially creating market concentration that stifles innovation.
How To Choose The Right Partner For Gen AI Advancement?
Selecting the right technology partner can determine whether AI drug discovery initiatives succeed or become expensive failures.
Here’s what organizations should evaluate when choosing Gen AI healthcare collaborators.
1. Technical Capabilities and Platform Maturity
Assess whether potential partners have production-ready platforms or just promising prototypes. Request case studies showing actual drug candidates advanced to clinical trials, not just computational validations.
Evaluate the sophistication of their generative models—are they using cutting-edge architectures or repackaging older approaches with AI buzzwords?
2. Data Assets And Quality
A partner’s proprietary datasets often matter more than their algorithms. Inquire about the size, diversity, and quality of their training data.
Do they have access to real-world clinical outcomes, or only preclinical research data? Is their genomic data representative of diverse populations?
Partners with established pharmaceutical collaborations often have access to superior datasets through those relationships. However, ensure data-sharing agreements allow them to leverage learnings from other clients to benefit your projects.
3. Regulatory Experience And Compliance
Choose partners with demonstrated regulatory success. Have they supported drugs through approval processes? Do they understand regional regulatory variations? Can they provide documentation that meets evolving AI validation requirements?
Partners should have robust processes for model explainability—the ability to show regulators why AI systems made specific recommendations. At HyScaler we have the expertise of understanding different compliances and guide with better solutions in AI/ML Solutions.
4. Integration And Interoperability
Evaluate how well partner solutions integrate with existing pharmaceutical development infrastructure. Can their platforms connect with your electronic lab notebooks, clinical trial management systems, and regulatory submission tools? Do they support standard data formats and APIs?
5. Transparency & Intellectual Property Terms
Clarify who owns AI-generated discoveries. Some partners claim rights to molecules their systems design; others allow clients full ownership. Understand whether you’re licensing software, purchasing services, or entering genuine research collaborations.
Conclusion
The transformation of personalized medicine through AI drug discovery represents one of the most significant healthcare advances of our generation.
In 2025, we’re witnessing the maturation of technologies that seemed speculative just years ago: generative models designing effective therapies, genomic AI predicting disease years before symptoms appear, and personalized treatment plans optimized for individual patient biology.
And, at HyScaler we make your dream come true with our efficient software solutions.
FAQ
Q1. How is AI used in drug discovery and development?
AI is used throughout drug discovery to accelerate multiple stages of development. Generative AI models design novel drug molecules by predicting how chemical structures will interact with disease targets. Machine learning algorithms screen millions of compounds to identify promising candidates, predict toxicity, and optimize pharmacological properties.
Q2. What are the main benefits of AI-powered genomics in personalized medicine?
AI-powered genomics enables precision diagnostics by identifying disease risks years before symptoms appear. It optimizes medication selection through pharmacogenomics—analyzing how individual patients metabolize drugs to predict efficacy and adverse reactions.
For rare diseases, AI identifies treatment opportunities that traditional economics would never pursue.
Q3. What challenges does AI face in precision medicine adoption?
Major challenges include fragmented healthcare data lacking standardization across institutions, regulatory uncertainty about validating AI-designed therapies, and algorithmic bias from training datasets that underrepresent diverse populations. Clinical integration difficulties arise when AI systems disrupt existing workflows or overwhelm providers with poorly designed recommendations.